What is a Fuel Cell?
Fuel Cell is an electrochemical device that is used to convert an open source fuel into electricity. An electrolytic process has to take place inside a cell in which there is an open source fuel [hydrogen] and an oxidant [oxygen]. Both the fuel and oxidant reacts in the presence of an electrolyte. Both the fuel and oxidant are introduced into the cell, where they react and the output product is carried out of the cell and stored. The electrolyte is left as it is inside the cell. This process can take place non-stop for a long time as long as the flow of resources are maintained.
The result obtained by combining hydrogen and oxygen is water. As a result of this process, electricity is formed. Although batteries are also electrochemical devices, they are different from a fuel cell. They use reactants from an external source and the chemicals have to be stored inside the battery. These chemicals react to each other to produce the electricity. Thus they use closed source fuel. As the device stores the required energy in a chemical form, the battery has to be recharged at intervals or have to be replaced.
Other than hydrogen the other types of fuels commonly used are hydrocarbons and even alcohol. The most commonly used oxidants are oxygen, chlorine and also chlorine dioxide.
Need of Fuel Cells
The main reason for the use of fuel cell is the increasing dependency on the use of fossil fuels. The whole world has burnt so much fossil fuel like oil to such an extent that they have become one of the main reasons for the pollution. This pollution has eventually resulted in the global warming and extreme climate change. Other than the environmental problems, the use of oil has become large enough that the sources of production have become less. As a result more challenging expeditions will have to be made for oil deposits which results in a very high oil price.
Fuel cells are surely an alternative to the above said problems. They are a clear solution to the dependency of fossil fuels. The best feature of fuel cells is that they produce pure water as the by-product. As a result they are pollution free as well.
As a part of making hydrogen fuel cell dependent vehicles possible in the most efficient and cheapest way, American president George Bush announced the Hydrogen Fuel Initiative program (HFI) in the year 2003. The country has also spent nearly one billion dollars for the research of better fuel cells. The technology will surely become practical soon and thus bring a solution to the rising oil problems.
Basic design and Working of a Fuel Cell
For any type of fuel cell, there are mainly three segments.
- Anode
- Cathode
- Electrolyte
The type of electrolyte used is what defines the type of fuel cell used. Whatever may be the type of fuel cell, their basic operation is always the same.
With the combination of the three segments, two main chemical reactions take place. A catalyst will be present at the anode. This anode catalyst, mostly platinum powder, is used to oxidise the hydrogen fuel. Thus the hydrogen gas turns into ions and electrons. Out of these, the ions make way through the electrolyte to the cathode. As soon as they reach the cathode, they combine with the cathode and then react with the oxidant to produce water. The electrons pass through a wire producing the electricity. Nickel is mostly used as the cathode catalyst. Thus the electricity is formed at the load and water is obtained as the by-product.
Though a fuel cell can normally produce only up to 0.7 volts at full load, the desired amount of voltage can be obtained by combining the fuel cells in series. For obtaining the desired amount of current, the fuel cells can be connected in parallel.
The fuel cell also has certain losses which causes a lesser amount of voltage to be produced at a higher current rate. Some of the losses are ohmic loss. Activation loss and also the loss due to the mass depletion of reactants called mass transport loss.
Different Types of Fuel Cells
The type of fuel cell differs mainly according to their operating temperature and the different electrolytes used. Some of the most common are given below.
Polymer Electrolyte Membrane Fuel Cell (PEMFC)
Polymer Electrolyte Membrane Fuel Cell (PEMFC) uses electro-chemical reaction to react both hydrogen and oxygen to form water as a by-product and also electricity. The device is said to be completely pollution free and with an efficiency of more than 50%. As the name suggests, in between the anode and cathode terminals is a sandwiched membrane called the proton-exchange polymer membrane. This will act as the electrolyte. This membrane can conduct only positive charged ions and stops electrons from passing through. To use this electrolyte in this device, the membrane should be in the hydrated form so as to be in the stable form.
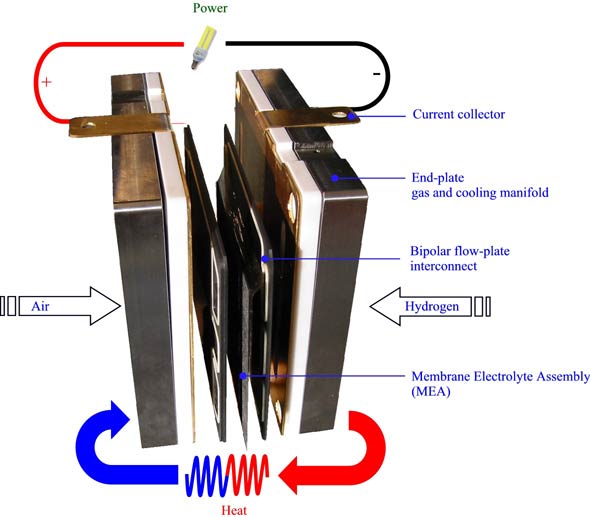
The anode, a cathode and the membrane are together called as the Membrane Electrode Assembly [MEA]. The earlier mentioned catalysts are used here as well. But the anode catalyst will be assembled in a carbon fibre substrate.
In the anode, the hydrogen gas reacts with the anode catalyst causing it to split into protons and electrons. The protons are then carried to the oxidant region where they react together form multi-facilitated proton membranes. The electrons travel through another wire and react with the oxygen as well as protons and thus water is produced. When the electron travels through the external circuit, electricity is also produced. Take a look at the diagram given below.
- Polymer Electrolyte Membrane Fuel Cell (PEMFC)
As usual the voltage produced will be very low [0.5 volts to 1 volt]. The wanted voltage can be obtained by adding fuel cells in series. This device is mostly efficient at low temperatures from 50 to 70 degree Celsius and also a high power density.
Alkaline fuel cell (AFC)
This type of fuel cell was been introduced since the early 1960’s. As the electrolyte used for this device is aqueous alkaline solution like potassium hydroxide, the procedure for electricity consumption is rather expensive.
Direct Methanol Fuel Cell (DMFC)
This device has somewhat the same characteristics as that of a Polymer Electrolyte Membrane Fuel Cell (PEMFC). But the only difference is in the percentage of efficiency. It has lesser efficiency [<30%] and also needs a huge amount of anode catalyst and thus highly expensive. The device uses Polymer membrane, mostly ionomer as the electrolyte.
Molten-Carbonate Fuel Cell (MCFC)
Molten alkaline carbonate like sodium bicarbonate is used as the electrolyte. They can produce high powers up to 100 Mega Watts. Thus they can be used as high power generators. They can also be operated at high temperatures up to 650 degree Celsius. They are not so expensive in production and hence can be used for commercial uses. It has an efficiency of almost 55%.
Phosphoric Acid Fuel Cell
Molten phosphoric acid is the electrolyte used in this type of fuel cell. It operates at high temperature up to 200 degree Celsius. It has an efficiency of up to 55%. This type of fuel cell is most commonly used in commercial cars.
Solid oxide Fuel Cell (SOFC)
This is one of the most commercially used fuel cell as they have the highest operating life. It has a very high operating temperature of 1,000 degrees Celsius. But other parts of the fuel cell may not be able to withstand at this temperature making it highly unstable. But, when used in a continuous state they can be highly reliable. At high temperatures the device can produce water in the form of steam which can be easily transported through steam turbines to produce more electricity, thus increasing the efficiency of the system. This device is also special in the case where a wide variety of fuels can be used. Most of the petroleum products can be used as the fuel. The electrolyte used in the cell is called yttria stabilized zirconia (YSZ). This electrolyte is good for large scale power generation and has the same characteristics as all the other electrolytes.
As the device has a very high operating temperature, there are some disadvantages as well. There may be unwanted number of reactions taking place inside the cell due t the high temperature. As a result of these reactions carbon dust and also graphite may be built up on the anode making it insufficient from reacting with the catalyst.
Fuel Cells – Advantages
- It is compact, light weight and has no moving parts. Thus it is 99.9% reliable.
- Pollution is reduced by 99%. This is the lowest pollution rate when compared to batteries as well as gasoline powered devices.
- If the device is used to power cars, it means that the efficiency level of all the three components will be different. Though the basic components of the car like tyre, transmission and so on are the same the efficiency is define on the power produced and the power converted to mechanical power. In the beginning almost 80% efficiency is produced by converting hydrogen into electricity. When this electricity is converted to mechanical energy to run the device, motor/motors and also an inverter will be needed. This will also have a efficiency of 80%. Thus the overall efficiency will be almost 65%, which is a high efficiency when compared to battery and gasoline. The overall efficiency of a battery is considered to be 60% and that of a gasoline powered vehicle is 40%.
Fuel Cells –Disadvantages
1. Cost
The overall production cost of a fuel cell is very costly. The anode catalysts like platinum and also gas diffusion layers almost hold up to 75% of the total cost. When compared to batteries and gasoline powered vehicles, they tend to be the costliest. If a kilowatt of power produced by the fuel cell comes around $35 to $40, it can be used. Currently, it costs up to $75. This can be done only by extensive research in replacing platinum with some other cheaper substance.
2. Durability
Most of the fuel cells that are used in cars, like PEMFC does not operate well enough in higher temperatures. As a result they have less tolerance level and less stability under running conditions.
3. Bad infrastructure
In order to make vehicles with fuel cells enough amount of hydrogen has to be generated. After generation process, they must also be carefully transported from the generating plants. This can be done only by transportation or pipelines. For this a proper infrastructure has not yet been developed.
Fuel Cells – Applications
- Can be used as power sources in remote areas.
- Can be used to provide off-grid power supplies.
- Can be applicable in both hybrid and electric vehicles.

2 Comments
really superb
really goood